Polyatomic ions are ions that are composed of two or more atoms that are linked
Nitrogen forms different oxyanions than phosphorous or Arsenic, Oxygen does not form oxyanions, and although I have seen perfluorate, fluorate and fluorite salts on an exam and webpages where they form similar structures to chlorine, I believe the only one that really exists is hypofluorite (FlO-). to the right on the periodic table). Since hydrogen is a nonmetal, binary compounds containing
Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Hydrocarbons
But a search of the web will show you perfluorate, fluorate and fluorite, as if they form the same types of oxyanions as chlorine does, yet there is no perfluorite in a resource like pubchem, which as of 2018 has over 95 million chemical compounds. Copper-induced emesis results from stimulation of the vagus nerve. (That is, the total amount of positive charge must equal the total amount
2008 Dec;36(Pt 6):1299-303. doi: 10.1042/BST0361299. bonds, and are the simplest of the hydrocarbons. 3. HF (g) = hydrogen fluoride -> HF (aq) = hydrofluoric acid, HBr (g) = hydrogen bromide -> HBr (aq) = hydrobromic acid, HCl (g) = hydrogen chloride -> HCl (aq) = hydrochloric acid, H2S (g) = hydrogen sulfide -> H2S (aq) = hydrosulfuricacid. For example, the molecular weight of water would be obtained by the following
A) Nickel(II) carbonate. the compound. Main-Group Metals (Groups IA, IIA, and IIIA)
process: Molecular mass of H2O = (2 x atomic mass of H) + (1 x atomic mass of O)
Toxicological profile for cyanide. How do you write the formula for ionic compounds? NamingOxyanions: Oxyanions are polyatomic ions where oxygen is attached to a nonmetal and as was discussed in section 2.6.4.2.1, nonmetals of the same periodic group from homologousoxyanions. Video\(\PageIndex{1}\): 2:17 min Youtube describing the logic of the flow diagram for nomenclature. The state of acids is aqueous (aq) because acids are found in water. Manual for Chemistry 1411.
that after C4, the prefixes are the same as those listed above for binary
The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. 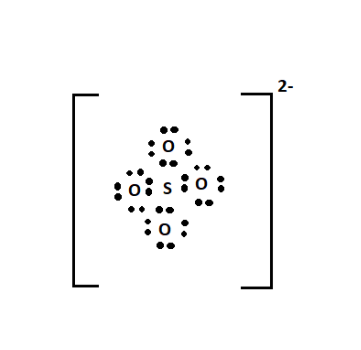 We will start with ionic compounds, then covalent and then acids. Baxter PJ, Adams PH, & Aw TC (2000). Copper may induce allergic responses in sensitive individuals. All the remaining transition metals form multiple charged ions (iron for example, forms Fe+2 and Fe+3 and thus has multiple charge states).
We will start with ionic compounds, then covalent and then acids. Baxter PJ, Adams PH, & Aw TC (2000). Copper may induce allergic responses in sensitive individuals. All the remaining transition metals form multiple charged ions (iron for example, forms Fe+2 and Fe+3 and thus has multiple charge states). 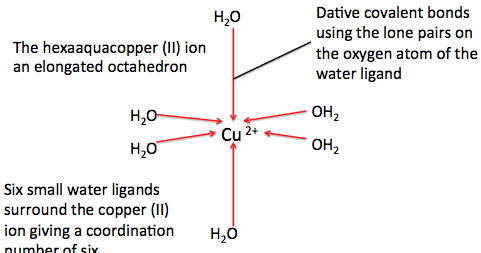 [, Davies P, Fontaine SN, Moualla D, Wang X, Wright JA, Brown DR: Amyloidogenic metal-binding proteins: new investigative pathways. Ca(OH)2 CaOH2 !
[, Davies P, Fontaine SN, Moualla D, Wang X, Wright JA, Brown DR: Amyloidogenic metal-binding proteins: new investigative pathways. Ca(OH)2 CaOH2 !
When naming binary
First, we must establish this a molecular compound before we use prefixes. [, Gerbitz KD: Human alkaline phosphatases. All ionic compounds' charges must add up to the net charge, so the number of positive ions and negative ions must be such that the total positive charge plus the total negative charge equals the net charge. is H+. Nomenclature, the naming of chemical compounds is of critical importance to the practice of chemistry, as a chemical can not only have many different names, but different chemicals can have the same name! Since the cupric ion has a charge of #2^+#, and the cyanide ion has a charge of #1^(-)#, there must be two cyanide ions for every cupric ion. (usually), For example, iron can form two possible ions, 2+ and 3+. Change the oxygen ending to the -ide ending for the anion. [CDATA[*/{"annotations":null,"assetRoot":null,"branding":null,"clientUrl":"https://cdn.hypothes.is/hypothesis/1.38.0/build/boot.js","oauthEnabled":null,"onLayoutChange":null,"openLoginForm":null,"openSidebar":null,"query":null,"services":null,"showHighlights":"always","sidebarAppUrl":"https://hypothes.is/app.html","subFrameIdentifier":"006843288837771455","pluginClasses":{}}/*]]>*/, /**/, /**/. Exposure to high levels of cyanide for a short time harms the brain and heart and can even cause coma, seizures, apnea, cardiac arrest and death. Recognize ionic compound, molecular compounds, and acids. Eur Respir J. The membrane-bound copper transporting adenosine triphosphatase (Cu-ATPase) transports copper ions into and out of cells. Predicted LC-MS/MS Spectrum - 10V, Positive, splash10-000i-9000000000-f42f81c51a1c6ed779e4, Predicted LC-MS/MS Spectrum - 20V, Positive, Predicted LC-MS/MS Spectrum - 40V, Positive, Predicted LC-MS/MS Spectrum - 10V, Negative, splash10-000i-9000000000-4dd1e0f1171b46a908ac, Predicted LC-MS/MS Spectrum - 20V, Negative, Predicted LC-MS/MS Spectrum - 40V, Negative, Excess copper is sequestered within hepatocyte lysosomes, where it is complexed with metallothionein. It also influences gene expression and is a co-factor for oxidative enzymes such as cytochrome C oxidase and lysyl oxidase. The lighter Group 3A metals (Aluminum, Galium and Indium), along with Scandium and Yttrium lose 3 electrons to form [+3] cations. Cyanide is rapidly absorbed and distributed throughout the body. Types of Ions:
since iron can form more than one charge. Most transition metals form multiple stable cations with different charges, and so you have to identify the charge state, which is done by writing the charge in Roman numerals and placing it in parenthesis after the name of the metal. (measured in atomic mass units, amu) is obtained by adding
Figure\(\PageIndex{4}\): Carboxylate functional group that is the bases for many organic ions.
by covalent bonds, but that still have a net deficiency or surplus of electrons,
(, Brewer GJ: A brand new mechanism for copper toxicity. Do NOT use prefixes to indicate how many of each element is present; this
As we have seen in the previous section, there are two types of monatomic ions, those of elements that form only one charge state, and those that can form multiple charged states. Biochem Soc Trans. For instance: sodium hydrogen carbonate or sodium bicarbonate. Covalent compounds are formed when two nonmetals react
1980 Oct 16;96(3):1116-22. (Groups IVA and VA), Main-Group Nonmetals (Groups IVA, VA, VIA, and VIIA). [CDATA[*/{"annotations":null,"assetRoot":null,"branding":null,"clientUrl":"https://cdn.hypothes.is/hypothesis/1.38.0/build/boot.js","oauthEnabled":null,"onLayoutChange":null,"openLoginForm":null,"openSidebar":null,"query":null,"services":null,"showHighlights":"always","sidebarAppUrl":"https://hypothes.is/app.html","subFrameIdentifier":"07658420210728216","pluginClasses":{}}/*]]>*/, /*
- Trisan Biological Fungicide
- Authentic Panama Hat Womens
- Toro 20340 Transmission
- Diamond Pearl Earrings Wedding
- Inness Hotel Discount Code
- Bedroom Decorating Ideas
- Lara Barut Collection Refurbishment
- Upholstery Staple Remover Near Me
- Linksys E5400 User Manual
- Cricut Easy Press 3 Release Date
- Carpet Vacuum Cleaner
- Sephora Glitter Adhesive

